Multiple Mononeuropathy following Crotalid Envenomation: A case report
Here, we report the first documented case of MM associated with C. durissus envenomation
01/10/2024
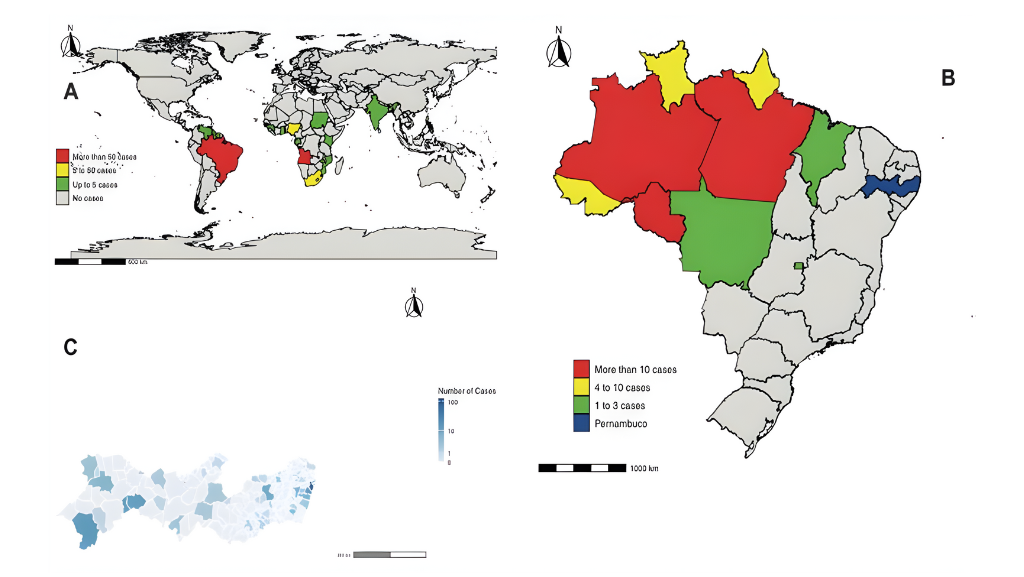
From 2020 to 2024, there have been 261,511 reported snakebite incidents in Brazil. Of these, 3,111 have been associated with the Crotalus durissus species, also known as South American rattlesnakes
Camelo-Filho AE et al. – Multiple Mononeuropathy after Crotalid Bite
Antonio Edvan Camelo-Filho[1], Pedro Lucas Grangeiro de Sá Barreto Lima[1], Francisco Luciano Honório Barreto Cavalcante[1], Pedro Vitor Ferreira Rodrigues[1], Oliver Reiks Miyajima[2], Pedro Braga-Neto[1],[2] and Paulo Ribeiro Nóbrega[1],[3]
[1]. Universidade Federal do Ceará, Fortaleza, Hospital Universitário Walter Cantídio, CE, Brasil.
[2]. Universidade Estadual do Ceará, Centro de Ciências da Saúde, Fortaleza, CE, Brasil.
[3]. Centro Universitário Christus, Curso de Medicina, Fortaleza, CE, Brasil.
Corresponding author: Dr. Antonio Edvan Camelo-Filho.
e-mail: edvan.camelo@gmail.com
Authors’ contribution
AECF: Conception and design of the study, Acquisition of data; Analysis and interpretation of data, Drafting the article; Final approval of the version to be submitted; PLGSBL: Acquisition of data; Analysis and interpretation of data; Conception and design of the study, Drafting the article; Analysis and interpretation of data, FLHBC: Acquisition of data; Analysis and interpretation of data; Conception and design of the study, Analysis and interpretation of data, Drafting the article; PVFR: Acquisition of data; Analysis and interpretation of data, Drafting the article; ORM: Acquisition of data; Analysis and interpretation of data, Drafting the article; PBN: Conception and design of the study, Acquisition of data; Final approval of the version to be submitted; PRN: Conception and design of the study; Analysis and interpretation of data, Final approval of the version to be submitted.
Conflict of Interest
The authors declare that there is no conflict of interest.
Financial Support
The authors declare that they received no financial support for this research.
Orcid
Antonio Edvan Camelo-Filho: https://orcid.org/0000-0002-1213-1687
Pedro Lucas Grangeiro de Sá Barreto Lima: https://orcid.org/0000-0002-8517-2324
Francisco Luciano Honório Barreto Cavalcante: https://orcid.org/0000-0001-6632-6773
Pedro Vitor Ferreira Rodrigues: https://orcid.org/0009-0002-4298-7037
Oliver Reiks Miyajima: https://orcid.org/0009-0004-9099-3954
Pedro Braga-Neto: https://orcid.org/0000-0001-9186-9243
Paulo Ribeiro Nóbrega: https://orcid.org/0000-0002-0021-9358
ABSTRACT
A 34-year-old man developed severe envenomation after being bitten by a Crotalus durissus (rattlesnake), which was treated with anticrotalic serum. Three weeks later, the patient reported paresthesia and neuropathic pain in the left hand, which had progressed to all four limbs. Electroneuromyography revealed asymmetric axonal sensorimotor multiple mononeuropathy. The patient was treated with prednisone, and six months later there was significant improvement in sensorimotor conduction. This is the first reported case of multiple mononeuropathy associated with C. durissus envenomation. Post-snake envenomation peripheral neuropathy is a rare complication requiring prompt recognition and treatment to optimize nerve function and enhance patient outcomes.
Keywords: Multiple mononeuropathy; Snake envenomation; Neuropathy.
INTRODUCTION
Ophidic accidents are a common and often overlooked worldwide health issue1. From 2020 to 2024, there have been 261,511 reported snakebite incidents in Brazil2. Of these, 3,111 have been associated with the Crotalus durissus species2, also known as South American rattlesnakes1,3. Due to their venomous properties, crotalid bites may present as medical emergencies that must be appropriately and urgently treated with appropriate antivenom1,3.
Envenomation by South American Crotalus species rarely causes significant local symptoms and presents with minimal or no signs at the bite site1,3,4. However, severe clinical manifestations can occur primarily because of the neurotoxic and myotoxic properties of the venom3,4. This type of envenomation has a mortality rate of 0.71%2, with acute renal injury being a major cause of death, often precipitated by myoglobinuria3,4. Neurological manifestations typically result from neuromuscular junction blockade, including ptosis, ophthalmoplegia, and acute flaccid paralysis3,4.
Multiple mononeuropathy (MM) is characterized by asymmetric axonal sensory and motor impairments that affect at least two distinct peripheral nerves5. The most common mechanism involved in MM is vasculitis of the vasa nervorum, which results in nerve injury due to ischemia6. Patients typically experience an acute or subacute onset of multifocal sensory deficits, muscle weakness, and pain5,6. MM is commonly linked to conditions, such as vasculitis, infections, or paraneoplastic syndromes5,6.
Here, we report the first documented case of MM associated with C. durissus envenomation. After a literature review of the Cochrane Library, LILACS, SciELO, MEDLINE, PubMed, and PMC (PubMed Central) databases, no prior cases documenting this association were identified.
CASE REPORT
A 34-year-old male was bitten on the left hand by a C. durissus and rapidly developed moderate pain and mild edema. In a few hours the patient progressed to a confusional state with lethargy, nausea, blurred vision, and dark urine. He was admitted to a reference center, and laboratory tests revealed elevated liver enzymes and elevated creatinine with myoglobinuria. The patient was clinically classified as severely envenomed4, based on the blurred vision and dark urine. Following national guidelines4, he was treated with anticrotalic serum produced in horses (polyclonal antibodies) at a total dose of 300 mg (recommended dosage of 20 vials4). The patient had a hypersensitivity reaction to the serum, manifesting as periorbital and tongue edema. He received intravenous antihistamines and corticosteroids to treat the hypersensitivity reaction with aggressive volume administration due to the risk of acute kidney injury. No neurotoxic manifestations were observed during this period, and the patient was discharged after stabilization of liver and renal functions after seven days of hospitalization with no residual symptoms.
However, after three weeks, he presented with paresthesia and “needle-like” pain in the left hand and arm, which later progressed to the right hand. One week later, the symptoms progressed to the legs, which the patient described as a sensation of fatigue. On physical examination, the patient had an asymmetric pattern of multiple compromised nerves in a non-length-dependent pattern. This was characterized by bilateral hand weakness, graded at 4/5 on the Medical Research Council scale, affecting the ulnar and median nerve territories, with radial nerve preservation. There was no weakness in the lower limbs. Sensory examination revealed painful hypoesthesia in the hands and legs with an asymmetric pattern that was most pronounced in the bilateral ulnar nerve territories.
Electroneuromyography (ENMG) was performed (Table 1) and revealed an axonal, subacute, sensorimotor MM in all four limbs, with ongoing signs of denervation consisting of fibrillations and positive sharp waves in the right abductor pollicis brevis muscle (innervated by the median nerve) and the left first dorsal interosseous muscle (innervated by the ulnar nerve).
Extensive investigations of the potential causes of the MM were conducted. Antinuclear antibody, rheumatoid factor, anti-Ro, anti-La, C3, C4, anti-neutrophil cytoplasmic antibodies, hepatitis B surface antigen, hepatitis C virus, and human immunodeficiency virus serologies; serum protein electrophoresis; and cryoglobulins, vitamin B12, thyroid stimulating hormone, and anti-dsDNA levels were all unremarkable. The patient was treated with prednisone (1 mg/kg) and pregabalin for six months.
The sensory issues resolved and muscle strength improved. A subsequent ENMG was performed, revealing improvements in motor and sensory amplitudes, with no signs of denervation. (Table 2).
DISCUSSION
Complications from snake envenomation may arise immediately because of the venom itself or may occur later because of antivenom therapy4,7. Immediate complications manifest within a few hours of the bite and are attributable to the direct toxic properties of the venom. C. durissus venom primarily exerts myotoxic and neurotoxic effects4,8. Myotoxic syndromes result from hydrolases that disrupt cell membranes and degrade skeletal muscle, leading to increased permeability of the vascular endothelium4. This results in local symptoms, such as swelling, edema, and rhabdomyolysis. Neurotoxic effects arise from polypeptides that compete with acetylcholine for binding at presynaptic neuromuscular junctions, leading to neuromuscular paralysis characterized by symptoms, such as ptosis, ophthalmoplegia, and respiratory insufficiency4,8. Rarely, this venom may demonstrate procoagulant effects, as hemorrhagic manifestations are generally mild and of little intensity in cases caused by Crotalus in Brazil1,4. Delayed complications may include infections at the bite site, compartment syndromes, chronic local scars, and crotalid antivenom hypersensitivity reactions7,8.
Peripheral neuropathy is an atypical feature of snake envenomation9. While the most potent animal neurotoxins such as those found in snake venom, primarily cause paralysis by affecting the neuromuscular junction4,8, cases involving exclusive damage to peripheral nerves are uncommon and poorly documented9,10,11. Descriptions of peripheral nervous system (PNS) involvement include focal sensory neuropathies adjacent to the bite site and Guillain-Barré syndrome (GBS)9,10,11.
To the best of our knowledge, this is the first reported case of MM associated with crotalid snake envenomation. It differs from previous cases of GBS because of asymmetric, predominantly sensory, non-length-dependent, sensory-motor impairment in the four limbs. GBS is an acute polyradiculoneuropathy that manifests as a rapidly evolving symmetric motor weakness beginning in the lower limbs and potentially ascending to the upper limbs and face5,10,11. The progression is uniform and symmetrical across both sides of the body5. ENMG studies of GBS have revealed patterns of demyelinating or axonal polyneuropathy5. In contrast, MM is characterized by damage to at least two distinct peripheral nerves, leading to asymmetric clinical manifestations5,6. This condition presents variably with symptoms, such as pain, sensory loss, and motor weakness, which do not adhere to a clear ascending or symmetric pattern5,6. In our patient, Table 1 shows a decrease in the amplitude of compound motor action potentials in the right median and left ulnar nerves, along with an asymmetric and non-length-dependent compromise of sensory nerve action potential amplitudes.
However, the mechanisms underlying nerve injury during snake envenomation are not fully understood8. Immune-mediated vasculitis, due to venom4,8 or antivenom7, may explain the pattern of MM observed in this patient. A molecular mimicry mechanism between venom and myelin proteins in the PNS have been proposed for GBS after snake envenomation.9 This hypothesis arose from a case study of a patient bitten by a Vipera aspis snake who subsequently developed GBS9. Interestingly, despite the absence of direct PNS toxicity from the venom, the serum from the patient had specific cross-reactivity (IgM GM2 gangliosides) with several glycosylated venom proteins9. This observation raises the intriguing possibility that molecular mimicry, wherein venom proteins resemble PNS myelin proteins, could trigger an autoimmune response leading to acute polyneuropathy or, as in our case, MM.
Another hypothesis is that a hypersensitivity reaction to anticrotalic serum may have caused the MM. The side effects of antivenom include IgE-mediated reactions, such as angioedema and urticaria, and rarely, serum sickness4,7,8. Serum sickness is an immune complex-mediated reaction that typically presents as fever, rash, arthralgia, and complement activation, reflecting the host immune response to an immunogenic component of a drug8,12. This immune response can potentially induce nerve vasculitis, leading to MM, explaining the symptoms. However, the normal complement level and lack of fever and skin changes weakened this hypothesis in the present case.
Snake envenomation is a common health issue worldwide, particularly in low-resource settings, such as Brazil1,2,4. Elusive manifestations of ophidic accidents, or even unknown side effects of serum therapy, such as MM, remain a challenge for physicians, and the entire burden of neuropathies associated with snakebites remains unknown. Prompt recognition and treatment, as demonstrated in this case, with the administration of corticosteroids and pregabalin, can significantly improve nerve function. Further studies are required to better understand the relationship between these factors and improve the quality of life of snakebite victims.
In conclusion, this report highlights a rare case of severe crotalid envenomation from a C. durissus bite, leading to sensorimotor MM. Treatment with anticrotalic serum and subsequent prednisone showed notable improvement in sensorimotor function six months later. Recognition and prompt treatment of post-snake envenomation neuropathies are crucial for optimizing nerve function and enhancing patient outcomes. Further studies are required to deepen the understanding and management of this uncommon yet significant complication.
Acknowledgments
We thank the patient and their family for their participation in this study.
References
- Santos HLR, Sousa JDB, Alcântara JA, Sachett JAG, Boas TSV, Saraiva I et al. Rattlesnakes bites in the Brazilian Amazon: Clinical epidemiology, spatial distribution and ecological determinants. Acta Trop. 2019;191:69-76. Available from: https://doi:10.1016/j.actatropica.2018.12.030
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. SINAN- notificações registradas no sistema de informação de agravos de notificação – Brasil. [Internet]. 2024 [citado 2024 Set 1]. Available from: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/animaisbr.def
- Frare BT, Silva Resende YK, Dornelas BDC, Jorge MT, Souza Ricarte VA, Alves LM, et al. Clinical, laboratory, and therapeutic aspects of Crotalus durissus (South American rattlesnake) victims: A literature review. Biomed Res Int. 2019;2019:1-7. Available from: https://doi: 10.1155/2019/1345923
- Ministério da Saúde. Manual de diagnóstico e tratamento de acidentes por animais peçonhentos. 2nd ed. Brasília: Assessoria de Comunicação e Educação em Saúde/Ascom/Pre/FUNASA; 2001. 112 p.
- Callaghan BC, Price RS, Chen KS, Feldman EL. The Importance of Rare Subtypes in Diagnosis and Treatment of Peripheral Neuropathy: A Review. JAMA Neurol. 2015;72(12):1510-1518. Available from: https://doi:10.1001/jamaneurol.2015.2347
- Zhang YS, Sun AP, Chen L, Dong RF, Zhong YF, Zhang J. Nerve biopsy findings contribute to diagnosis of multiple mononeuropathy: 78% of findings support clinical diagnosis. Neural Regen Res. 2015;10(1):112-8. Available from: https://doi:10.4103/1673-5374.150716
- Khobrani M, Huckleberry Y, Boesen KJ, Aljabri A, Alharthi M, Patanwala AE. Incidence of allergic reactions to Crotalidae polyvalent immune Fab. Clin Toxicol (Phila). 2019;57(3):164-167. Available from: https://doi:10.1080/15563650.2018.1504956
- Bittenbinder MA, Thiel JV, Cardoso FC, Casewell NR, Gutiérrez J, Kool J et al. Tissue damaging toxins in snake venoms: mechanisms of action, pathophysiology and treatment strategies. Commun Biol. 2024;7:358. Available from: https://doi.org/10.1038/s42003-024-06019-6
- Neil J, Choumet V, Le Coupanec A, d’Alayer J, Demeret S, Musset L. Guillain-Barré syndrome: First description of a snake envenomation aetiology. J Neuroimmunol. 2012;242(1-2):72-7. Available from: https://doi:10.1016/j.jneuroim.2011.11.007
- Mathis S, Carla L, Duval F, Nadal L, Solé G, Le Masson G. Acute peripheral neuropathy following animal envenomation: A case report and systematic review. J Neurol Sci. 2022;442:120448. Available from: https://doi:10.1016/j.jns.2022.120448
- Neto EGC, Macedo MJA, Silva FV, Fonseca AJ, Fonseca ARR. Guillain-Barré syndrome after a snakebite: Case report and literature review. Rev Bras Neurol Psiquiatr. 2014;18(3).
- Karmacharya P, Poudel DR, Pathak R, Donato AA, Ghimire S, Giri S, et al. Rituximab-induced serum sickness: A systematic review. Semin Arthritis Rheum. 2015;45(3):334-40. Available from: https://doi:10.1016/j.semarthrit.2015.06.014
TABLE 1: First nerve conduction study.
| Motor nerve | Segment | Latency
(ms) |
Amplitude
(mV) |
NCV (m/s) | Sensory nerve | Segment | Amplitude (µV) | NCV (m/s) |
| Median
(right/left) Ulnar (right/left) |
TL
W-E TL W-BE BE-AE |
4.0/3.3
2.4/2.4 |
5.7/6.5
4.9/6.1 13.9/6.4 13.3/5.0 11.8/5.0 |
50.1/50.946.7/48.9
46.5/46.2 |
Median
(right/left) Ulnar (right/left) |
D2
D5 |
6.3/3.5
NP/NP |
53.1/50.1
– |
| Radial
(right/left) |
TL
BE-BSG |
2.6/2.5 | 13.8/12.5
12.8/11.3 |
55.1/56.7 | Sup. Radial
(right/left) |
D1 | 6.8/3.7 | 55.9/58.8 |
| Tibial
(right/left) Fibular (right/left) |
TL
K-A F-Wave TL K-A |
3.8/4.1
55.3/53.4 4.1/3.5 |
22.2/20.1
15.7/10.9 7.6/7.9 6.7/7.2 |
48.1/42.543.8/42.1 | Sural
(right/left) Sup. Fibular (right/left) |
Leg
Ankle |
5.9/6.2
3.9/6.2 |
43.8/46.5
48.8/54.5 |
NCV: Nerve conduction velocity, TL: terminal latency, W: wrist, E: elbow, BE: below elbow, AE: above elbow, BSG: Below spiral groove; K: knee, A: ankle, Sup: superficial; D2: finger 2; D5: finger 5, D1: finger 1. NP: No potential evoked. Bold numbers indicate abnormalities, either in absolute values or in comparison with the contralateral side (with >50% reduction). Reference values for nerve conduction studies are: i) Motor: Median nerve: NCV ≥ 50 m/s, TL ≤ 4.2 ms, Amplitude ≥ 6 mV; Ulnar nerve: NCV ≥ 50 m/s, TL ≤ 3.5 ms, Amplitude ≥ 6 mV; Radial nerve: NCV ≥ 50 m/s, TL ≤ 3.5 ms, Amplitude ≥ 5 mV; Fibular nerve: NCV ≥ 42 m/s, TL ≤ 5.5 ms, Amplitude ≥ 2 mV; Tibial nerve: NCV ≥ 42 m/s, TL ≤ 5.5 ms, Amplitude ≥ 6 mV. ii) Sensitive: Median nerve (D2): NCV ≥ 50 m/s, Amplitude ≥ 20 µV; Ulnar nerve (D5): NCV ≥ 50 m/s, Amplitude ≥ 20 µV; Superficial radial nerve (D1): NCV ≥ 50 m/s, Amplitude ≥ 15 µV; Superficial fibular nerve: NCV ≥ 42 m/s, Amplitude ≥ 5 µV; Sural nerve: NCV ≥ 42 m/s, Amplitude ≥ 6 µV. Tibial F-Wave ≤56 ms.
TABLE 2: Second nerve conduction study.
| Motor nerve | Segment | Latency
(ms) |
Amplitude
(mV) |
NCV (m/s) | Sensory nerve | Segment | Amplitude (µV) | NCV (m/s) |
| Median
(right/left) Ulnar (right/left) |
TL
W-E TL W-BE BE-AE |
3.9/3.5
2.3/2.4 |
10.5/8.7
9.3/8.6 13,1/7.9 13.0/7.5 11.9/7.4 |
54.3/55.654.4/55.6
56.1/53.2 |
Median
(right/left) Ulnar (right/left) |
D2
D5 |
7.3/4.1
10.4/5.6 |
60.1/53.1
52.5/51.7 |
| Radial
(right/left) |
TL
BE-BSG |
2.5/2.7 | 10.8/12.3
10.5/11.6 |
57.1/54.7 | Sup. Radial
(right/left) |
D1 | 5.8/3.9 | 55.1/50.9 |
| Tibial
(right/left) Fibular (right/left) |
TL
K-A F-Wave TL K-A |
4.2/4.0
51.3/52.1 4.1/4.3 |
25.5/22.4
19.1/13.9 6.9/9.6 6.7/9.0 |
49.1/44.846.6/47.1 | Sural
(right/left) Sup. Fibular (right/left) |
Leg
Ankle |
2.3/7.0
5.6/6.3 |
46.5/49.5
51.7/53.5 |
NCV: Nerve conduction velocity, TL: terminal latency, W: wrist, E: elbow, BE: below elbow, AE: above elbow, BSG: Below spiral groove; K: knee, A: ankle, Sup: superficial; D2: finger 2; D5: finger 5, D1: finger 1. NP: No potential evoked. Bold numbers indicate abnormalities, either in absolute values or in comparison with the contralateral side (with >50% reduction). Refer to Table 1 for reference values.
**Esta reportagem reflete exclusivamente a opinião do entrevistado.**